Conversion of sugar and formation of wort density in beer fermentation
Enzymatic sugar analysis | Accurate determination of the end of fermentation is a crucial step in controlling the brewing process. One of the main issues here is accurately assessing when fermentation ends. The secondary fermentation phase, with precise adjustment of the carbonation in the final fermented beer, must also be closely monitored [1].
Researchers at the “Francesco Bonicolini” CDR Chemical Lab carried out a study on beer production targeting the most reliable method of detecting end of fermentation and measuring the residual sugar concentration to avoid problems during the priming stage. With this objective in mind, conversion of fermentable sugars and changes in wort density during fermentation were investigated.
Sugars in beer wort
Four types of fermentable sugars may be present in beer wort [2]:
- glucose and fructose: simple sugars that can be fully fermented by yeasts used in brewing;
- maltose: a complex sugar comprising two glucose molecules, fully fermentable by yeasts;
- maltotriose: a complex sugar comprising three glucose molecules, fully fermentable by some brewing yeasts, while only partially or not at all fermentable by others;
- sucrose: a complex sugar comprising one glucose molecule and one fructose molecule, easily fermentable by yeasts.
Glucose, fructose, maltose and maltotriose are naturally present in beer wort as they are derived from barley starch during mashing. In this scenario, some sucrose has been added in order to analyse conversion of this sugar during fermentation.
Materials and methods
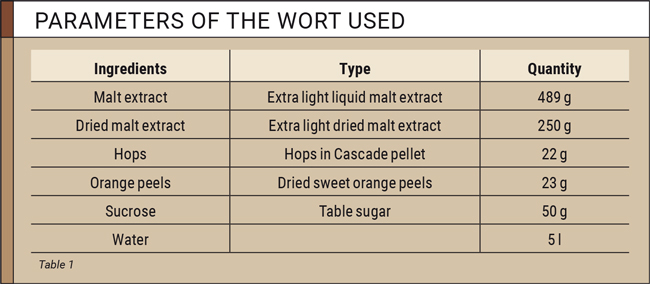
For this study, two beer worts were prepared in the CDR laboratory according to the recipe given in table 1.
One wort was fermented using the White Labs yeast WLP002, which achieves a fermentation rate of 63–70 percent. The second wort produced was fermented with a strong fermenting yeast, White Labs WLP099. This yeast achieves fermentation levels from 80 to 100 percent.
The parameters of the water used were as follows:
- Calcium: 115 mg/L;
- alkalinity: 98 ppm;
- sulphates: 256 mg/L;
- chlorides: 56 mg/L.
Yeasts used
The White Labs WLP002 yeast is a low attenuation yeast (63–70%), therefore not capable of converting all sugars into alcohol. The optimal temperature range for fermentation is 18–20°C, it has very high flocculation and a medium alcohol tolerance (5–10% v/v).
A slight production of diacetyl is common with WLP002. Due to the high degree of flocculation of this strain, the finished beer is clear and the yeast can be easily removed from the fermenter for future use.
White Labs WLP099 is a high attenuation yeast (80–100%), which is thus capable of converting almost all sugars into alcohol. The optimal temperature range for fermentation is 18–20.5°C, it has a medium degree of flocculation and a very high alcohol tolerance (>15% v/v). This yeast variety was genetically modified to achieve this high degree of fermentation. Data on the concentration of fermentable sugars and the density of the wort was collected from the time the yeast was added to the respective wort.
Measurement tools and analysis
The CDR BeerLab® was used to measure changes in concentration of fermentable sugars and a portable digital densimeter to measure changes in wort density. Data was collected from yeast inoculation until end of fermentation. For each analysis, about 10 mL of fermenting wort was taken from each container and filtered through paper filters. Approximately 3 mL of sample was used for density measurement. About 1 mL was sufficient to perform all analyses using CDR Beer Lab.
Results
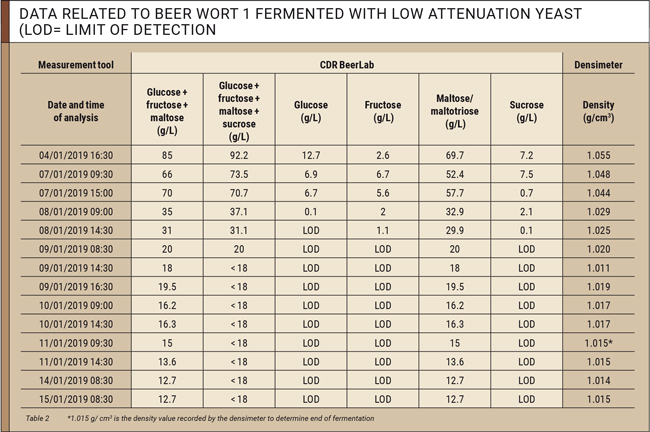
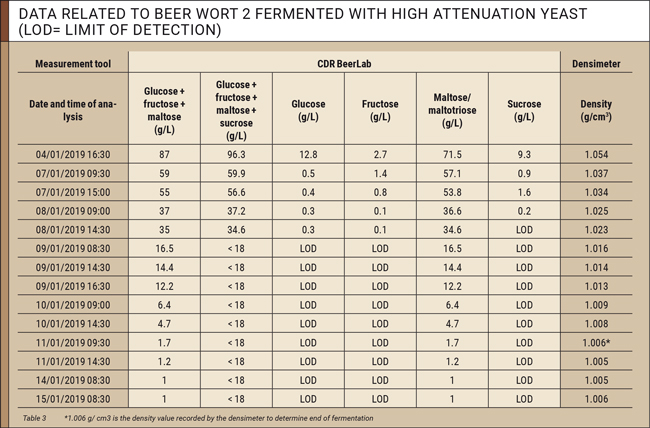
The data collected for each beer wort during fermentation is listed in tables 2 and 3.
The CDR BeerLab analysis system can perform two types of analysis: the determination of the sum of glucose, fructose and maltose and the determination of the sum of glucose, fructose, maltose and sucrose. However, in order to provide complete information, the various sugars were measured individually for this study.
The analysis system records glucose, fructose, maltose, maltotriose and sucrose levels enzymatically. The kit uses several highly specific enzymes for glucose and for the maltose/maltotriose pair, these are measured together, not separately. Fructose is determined based on isomerisation to glucose, whereas sucrose is converted to glucose using invertase and then analysed using the same enzymatic pathway as glucose. Fructose, derived from inversion of sucrose, is also isomerised to glucose and measured using the same enzymatic pathway as free glucose. The system, comprising an analytical instrument and an enzymatic kit, is calibrated and ready for use. In the case of the sugar determination kit, standards at various concentrations of glucose, fructose, maltose, maltotriose, and sucrose are used for calibration. The CDR BeerLab method for determination of sugars is enzymatic and is thus not affected by any interference or any matrix effects.
Based on data listed in tables 2 and 3, it is possible to assess the trend of concentrations of fermentable sugars in the beer wort. The first sugars fermented by yeasts are glucose, fructose, and sucrose. These three sugars are almost absent after four days in most worts. Subsequently, maltose and maltotriose (measured along with maltose) are fermented. Fermentation can be considered complete after 6 days when concentration of sugars remains constant in two measurements taken 24 hours apart.
When focussing on the column for glucose + fructose + maltose (g/L) in the tables, the yeasts behaved differently at the end of fermentation. When using e.g. the low attenuation White Labs WLP002 yeast, 12.7 g/L of unfermented sugar (maltotriose) remained in the wort, whereas the high attenuation White Labs WLP099 yeast fermented nearly all sugars, leaving only 1 g/L of unfermented sugar (maltotriose) in the wort. This difference in final sugar concentrations, resulting from the characteristics of the yeasts used, should be considered when assessing end of fermentation, as it shows that fermentation does not always end when the residual sugar concentration approximates zero. It is also important to know the residual sugar concentration at the end of fermentation in order to correctly assess the amount of sugar to be added during the priming phase to avoid over-carbonation.
Comparison of the methods used
To determine the end of fermentation and measure the residual sugar concentration, it is necessary to focus on the last four days of the process.
As shown in the tables, the value at the end of fermentation recorded by the densimeter is 1.015 g/cm3 for beer wort 1 (low attenuation yeast) and 1.006 g/cm3 for beer wort 2 (high attenuation yeast) respectively.
For both worts 1 and 2, wort density measured by the portable densimeter over the last four days varies by 0.001. As its resolution is 0.001, the densimeter provides a potentially insignificant result as it is not able to reflect the change in sugar concentration below measurement resolution.
However, analytical results obtained with CDR BeerLab in the last 4 days of fermentation show that concentrations of fermentable sugars measured in wort 1 still dropped 2.3 g/L and 0.7 g/L for wort 2.
- Wort 1: 15 g/L (1.015 g/cm3) – 12.7 (sugars at the end of fermentation measured by CDR BeerLab) = 2.3 g/L;
- Wort 2: 1.7 g/L (1.006 g/cm3) – 1.0 (sugars at the end of fermentation measured by CDR BeerLab) = 0.7 g/L.
These amounts of residual fermentable sugars would have resulted in 0.57 and 0.17 CO2 contents, potentially leading to excessive carbonation in the beer.
This data shows that the portable digital densimeter is quite accurate in assessing wort density and can thus be used to monitor the progress of fermentation. However, the CDR BeerLab results are much more effective to determine the actual end of fermentation than the portable densimeter as the former is able to detect even small changes in sugar concentration.
Adjusting carbonation levels
The evidence that fermentation has been completed is essential for the priming phase to prevent over-carbonation possibly triggering gushing.
Analysis of sugars is very important in the priming stage in order to obtain better control over the final fermentation in the bottle. In this way, the CO2 quantities needed for the respective recipe can be calculated.
Conclusions
It can be concluded that fermentation has ended when the sugar concentration remains constant for 24 consecutive hours. To determine the end of the fermentation process, it is thus much more effective to monitor the change in sugar concentration in the wort rather than density. CDR BeerLab proves to be the more suitable instrument for determining the end of fermentation compared to the portable densimeter, as it is able to show even the smallest changes in sugar concentration that would not be detected by density measurement.
To avoid errors during the priming phase, it is important to use an analysis system that can measure the exact concentration of residual sugars at the end of fermentation.
References
- Marconi, O.; Rossi, S.; Galgano, F.; Sileoni, V.: “Influence of yeast strain, priming solution and temperature on beer bottle conditioning”, Journal of Science and Food Agriculture, 96(12), 2016, pp. 4106–4115.
- Buckee, G. K.; Hargitt, R.: “Measurement of Carbohydrates in wort and beer a Review”, J. Inst. Brew., 84, 1978, pp. 13–21.
Keywords
fermentation gushing analytical methods fermentable sugars
Authors
Simone Bellassai, Lisa Mearelli
Source
BRAUWELT International 4, 2024, page 217-219
Companies
- CDR Foodlab "Francesco Bonicolini", Florenz, Italy